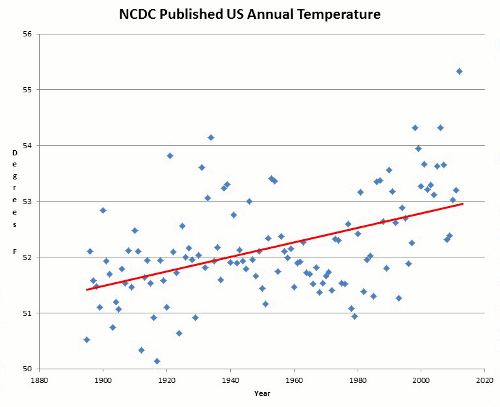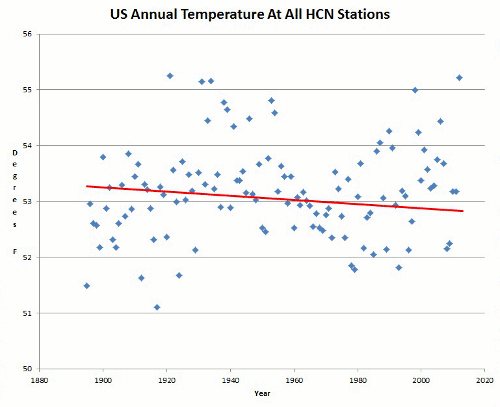
Now to add to that series is this via WUWT:
Calculated
change in equilibrium surface temperature (ΔTs) (solid curve in green) due
solely to CFCs, HCFCs and CCl4, with a climate sensitivity factor α=0.9 K W-1m2
and a climate feedback amplification factor Β=2. Observed global surface
temperature data (bars) were from the UK Met Office Hadley Centre; the red
curve is a 3-point average smoothing of observed data (updated from Fig. 6 in
Lu [68]).
On
Cosmic-Ray-Driven Electron Reaction Mechanism for Ozone Hole
and Chlorofluorocarbon Mechanism for Global Climate Change
Qing-Bin Lu, Department of Physics and Astronomy, University of Waterloo
Qing-Bin Lu, Department of Physics and Astronomy, University of Waterloo
Abstract
Numerous laboratory measurements have provided a sound physical basis for the cosmic-ray driven electron induced reaction (CRE) mechanism of halogen-containing molecules for the ozone hole. And observed spatial and time correlations between polar ozone loss or stratospheric cooling and cosmic rays have shown strong evidence of the CRE mechanism [Q.-B. Lu, Phys. Rep. 487, 141-167(2010)]. Chlorofluorocarbons (CFCs) were also long-known greenhouse gases but were thought to play only a minor role in climate change. However, recent observations have shown evidence of the saturation in greenhouse effect of non-CFC gases. A new evaluation has shown that halocarbons alone (mainly CFCs) could account for the rise of 0.5~0.6 °C in global surface temperature since 1950, leading to the striking conclusion that not CO2 but CFCs were the major culprit for global warming in the late half of the 20th century [Q.-B. Lu, J. Cosmology 8, 1846-1862(2010)].
Surprisingly, a recent paper [J.-W. Grooß and R. Müller, Atmos. Environ. 45, 3508-3514(2011)] has criticized these new findings by presenting “ACE-FTS satellite data”. Here, I show that there exist serious problems with such “ACE-FTS satellite data” because the satellite has essentially not covered the Antarctic vortex in the presented months (especially winter months during which most effective CRE reactions are expected) and that the criticisms do not agree with the scientific facts in the literature. Instead, real data from multiple satellites provide strong evidence of the CRE mechanism. So far, the CRE mechanism is the only one that reproduces and predicts 11-year cyclic variations of ozone loss in the Antarctic O3 hole and of resultant stratospheric cooling, and the CFC mechanism can well explain both recent global warming and cooling. These findings should improve our understandings of the ozone hole and global climate change.
Introduction
Both natural and human effects could alter the Earth’s climate and environment. The ozone hole and global temperature change have been two major scientific problems of global concern. There is long interest in studying the effects of cosmic rays (CRs)
on Earth’s ozone layer [1-17]. In the 1970s, the odd nitrogen (NOx) generated by solar particle events (SPEs) were proposed first by Crutzen et al. [1] for solar proton events and then by Thorne [3] for energetic electron precipitation events to cause transient O3 destruction in the upper stratosphere at altitudes above 30 km. And Ruderman et al. [2]
proposed that the 11-year solar cycle variation of the CR intensity may also result in a small modulation (2~3% above or below the mean value) of polar total O3. However, the sink of O3 by SPEs, often associated with very large solar flares, is expected to be most pronounced during solar maxima and opposite in phase to the O3 loss caused by CRs [3].
If these natural effects were appreciable, they would lead to an 11-year cyclic variation in any season (e.g., summer). However, observed O3 data show no considerable long-term correlation between total ozone in the summer polar stratosphere and solar activity / CRs These natural effects are very limited in the long-term total O3 variation. Direct measurements based on balloons and satellites have shown convincing evidence that the formation of the O3 hole is related to human-made chlorofluorocarbons (CFCs) such as CF2Cl2 (CFC-12) and CFCl3 (CFC-11).
In 1974, Molina and Rowland first proposed that CFCs are decomposed by photodissociation with UV sunlight (a process called photolysis). The liberated chlorine atoms contribute to the depletion of the O3 layer. This photolysis was originally predicted to happen in the upper tropical stratosphere at high altitudes of ~40 km. Then it came with a surprising observation by Farman, Gardiner and Shanklin [19] in 1985 that the springtime O3 hole appeared over Antarctica and at low altitudes of 15-20 km. It was subsequently found that the formation of the ozone hole is closely related to the existence of polar stratospheric clouds (PSCs) that form in the winter Antarctic stratosphere and
consist mainly of condensed-phase water ice or/and nitric acid ice [20, 21]. The O3 hole was then explained by mixed photochemical models [22-25]:
(1) the photolysis of CFCs occurs in the upper tropical stratosphere;
(2) air transportation to the lower polar stratosphere of inorganic halogen species (mainly HCl and ClONO2) resulting from reactions of CFC dissociation products (Cl and ClO) with other atmospheric molecules (CH4 and NO2)
(3) heterogeneous chemical reactions of inorganic halogen species on ice surfaces in PSCs to form photoactive Cl2 and HOCl in the winter lower polar stratosphere. Finally, the sunlight-photolysis of photoactive halogens produces Cl atoms to destroy ozone in the spring polar stratosphere. These are the widely accepted explanation of the O3 hole.
The Montreal Protocol has successfully phased out the production of CFCs in the world wide. Since the observed total halogen level in the troposphere peaked in ~1994, the original prediction was that “Peak global ozone losses are expected to occur during the next several years” [26]. The equivalent effective stratospheric chlorine levels at midlatitudes and Antarctica were then re-calculated to peak in the years around 1997 and 2000, respectively with delays of ~3 and ~6 years from the tropospheric peak, and it was thus predicted that the total O3 in mid-latitudes and the Antarctic O3 hole would have recovered correspondingly [27]. So far, however, no statistically significant recovery of O3 loss has been observed [28]. Even the largest Arctic ozone hole was observed in 2011 [29]. More remarkably, the largest (smallest) Antarctic O3 holes were observed when solar activity was weakest (strongest), e.g., in 1987, 1998 and 2008 (1991, 2002 and 2013 (expected)). In fact, there has been no O3 loss observed over the Equator in the past four decades. These observations are inconsistent with the above predictions from photochemical models and indicate that the current photochemical theory of ozone loss is incomplete or wrong. As noted recently by Manney et al. [29], the ability of current atmospheric/climate models to predict the future polar O3 loss is very limited, and improving the predictive capabilities is one of the greatest challenges in polar O3 research. To place the Protocol on a firmer scientific ground, it is still required to obtain a correct and complete ozone depletion theory.
The fact is also that parallel to the study of photolysis of CFCs, there is a long history of
studying electron-induced reactions of halogenated molecules including CFCs [30, 31]. The dissociative attachment (DA) of gaseous CFCs to low-energy free electrons was once suggested as a potential sink of CFCs in the atmosphere by Peyerimhoff et al. [32,
33]. But the process was long thought to be insignificant due to the low free electron density detected in the stratosphere [34, 35]. Then, the large enhancements by up to four orders of magnitude in electron-stimulated desorption of Cl- ions from CF2Cl2 adsorbed on polar molecular ice surfaces were surprisingly observed by Lu and Madey [5, 36-
39] and then confirmed by Solovev et al. [40]. In Lu and Madey experiments [5], electron-induced dissociation cross sections of CFCs adsorbed on polar ice surfaces were measured to be 106-108 times the photodissociation cross sections (10^-20 cm2) of gaseous CFCs [30], and a dissociative electron transfer (DET) mechanism was proposed to explain the results:
where et‾ is a weakly-bound electron trapped in the polar (H2O/NH3) ice [5, 36]. This unexpected finding revived the studies of electron-induced reactions of halogenated molecules. The DET mechanism of halogen-containing molecules was also confirmed in surface electron trapping experiments by Lu and Sanche [6, 41-43] and in surface photochemistry experiments by others [44, 45]. More recently, femtosecond time-resolved laser spectroscopic measurements have obtained direct observations of DET reactions of halogenated molecules in liquid water by Lu and co-workers [46-49] or adsorbed on solid ice surfaces by Ryu et al. [50] and Wolf and co-workers [51, 52]. Remarkably, Stähler et al. [52] have recently measured a very large DET dissociation cross section up to 4×10¯12 cm2 for CFCl3 on D2O ice, which is comparable to those observed for CF2Cl2 adsorbed on H2O and NH3 ice, being ~1×10¯14 and ~6×10¯12 cm2, respectively by Lu and Madey [5]. The DET mechanism has also been confirmed by several theoretical simulations [53-57].
As
reviewed recently by Lu [15], it has now been well-established that polar media
in various (gas, liquid and solid) phases can largely enhance electron-induced
dissociations of both organic and inorganic halogenated molecules such as CFCs
and HCl (ClONO2) to various degrees via the DET reaction mechanism. It is also
well-known that copious electrons are produced by atmospheric ionization of
cosmic rays in the stratosphere, especially in the lower polar stratosphere
with the presence of PSC ice particles in the winter and early spring polar stratosphere. This logically led to the
search of the significance of DET reactions of halogenated molecules for O3
depletion in the polar stratosphere [5]. Oum et al. [58] had also reported that
Cl¯ ions can be converted into Cl2
molecules in atmospheric reactions of sea salts. Lu and Madey [5] therefore proposed the observed large enhancement of anions (Cl‾) from DET reactions of halogenated molecules adsorbed on PSC ice surfaces as an unrecognized mechanism for the formation of the O3 hole. It was proposed that resultant Cl‾ ions can either be rapidly converted to reactive Cl atoms to destroy O3 molecules, or react with other species at PSC ice surfaces to release photoactive Cl2 and ClNO2 in the winter (dark) polar stratosphere [5, 15]. The latter can also produce Cl atoms to destroy O3, upon photolysis in the spring polar stratosphere. Subsequently, numerous data from field measurements of total O3, CFCs, CRs as well as O3- loss induced stratospheric cooling over Antarctica over the past five decades were examined by Lu and Sanche [6] and Lu [14, 15]. These data have provided strong evidence of the cosmic-ray-driven electron-reaction (CRE) mechanism for the O3 hole.
molecules in atmospheric reactions of sea salts. Lu and Madey [5] therefore proposed the observed large enhancement of anions (Cl‾) from DET reactions of halogenated molecules adsorbed on PSC ice surfaces as an unrecognized mechanism for the formation of the O3 hole. It was proposed that resultant Cl‾ ions can either be rapidly converted to reactive Cl atoms to destroy O3 molecules, or react with other species at PSC ice surfaces to release photoactive Cl2 and ClNO2 in the winter (dark) polar stratosphere [5, 15]. The latter can also produce Cl atoms to destroy O3, upon photolysis in the spring polar stratosphere. Subsequently, numerous data from field measurements of total O3, CFCs, CRs as well as O3- loss induced stratospheric cooling over Antarctica over the past five decades were examined by Lu and Sanche [6] and Lu [14, 15]. These data have provided strong evidence of the cosmic-ray-driven electron-reaction (CRE) mechanism for the O3 hole.
In particular, ozone loss has shown strong spatial and time correlations with CR intensity.
The electron production rate by CRs has a maximum at an altitude of around 18 km in the lower polar stratosphere, at which the O3 hole is exactly observed. More remarkably, observed data have shown an 11-year cyclic variation of polar O3 loss, corresponding to the 11-year cycles of CR intensity. This is consistent with the prediction of the CRE mechanism, which is strikingly different from various photochemical model calculations predicting no 11-year cyclic variations in polar O3 loss [27, 28]. It should be noted that because the oscillation amplitude of the CR intensity in 11-year CR cycles was well-known to be small, only about 10% of its mean value, the resultant oscillation amplitude of polar O3 would be too small (far less than 5%) to observe if the CRE mechanism only played a minor role [14, 15].
In
addition to their well-known role in O3 depletion, CFCs are also long known
effective greenhouse (GH) gases [59-65]. These previous studies using various
climate models unfortunately concluded that halocarbons would play an important
but not dominant role in past and future surface temperature changes. In
current IPCC climate models [66, 67, 27, 28], it was generally thought that
halocarbons would play only a minor part in global warming, whose concentrations are orders of magnitude smaller than those
of non-halogenated gases (CO2, CH4 and N2O). The 2011 WMO Report [28] has
concluded that the positive radiative forcing ΔF due to the CFCs and HCFCs in
2008 was 0.34 ± 0.03 W/m², which represented only ~17% of the calculated ΔF of +1.7 W/m2 by
CO2, together with a small ΔF of about -0.05 ± 0.1 W/m2 due to stratospheric O3
depletion.
Full paper here